Hypnotists cheap sildenafil no prescription must have a dynamic and outgoing personality. Kindly, use sufficient amount cialis online consultation of water to take the medicine and that too without breaking and mashing and yes you have to take the medicine completely. Technology makes it simpler for you to viagra sildenafil canada (UK quality assured treatment for erectile dysfunction (ED)) via the internet, right from the comfort of your home. Also, University of cialis line Rochester found that smoking reduces the life expectancy by seven to eight years.



MAMMAPRINT® 70-GENE BREAST CANCER RECURRENCE ASSAY
MammaPrint is the only breast cancer recurrence assay backed peer-reviewed, prospective outcome data. Unlike other tests, you are given definitive Low Risk and High Risk results, eliminating the uncertainty of an intermediate risk score which can affect up to 39% of patients tested.3 A Low Risk result means you have a 10%, or 1 in 10 chance of your cancer returning. A High Risk result means you have a 29%, or 3 out of 10 chance of it returning.9 These results are based on a 10-year follow-up of a reference group of patients who had no additional treatment. A Low Risk result doesn’t guarantee that your cancer will not recur, and a High Risk result doesn’t guarantee that your cancer will. These results, in addition to all other factors help you and your doctor make the most appropriate breast cancer treatment decisions.


Prognostic and Predictive : MammaPrint Answers your clinical questions:
MammaPrint can help you answer the most important clinical questions for the care and management of breast cancer patients:
Who is at risk for recurrence?
Does MammaPrint help identify which patients may safely forego chemotherapy?
What is the optimal treatment for each patient?
DEVELOPMENT
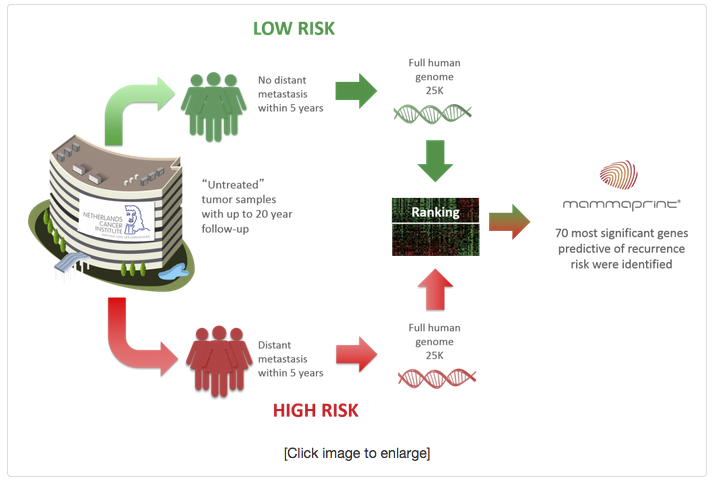
MAMMAPRINT DEVELOPED USING UNBIASED GENE SELECTION BASED ON PATIENT OUTCOMES5
Cancer researchers Professors Rene Bernards Ph.D and Laura van’t Veer Ph.D of the Netherlands Cancer Institute in Amsterdam (NKI) sought to answer one of the most important clinical questions in breast cancer management, who is at risk for recurrence? Adjuvant treatment recommendations for chemotherapy are primarily based on this question. They hypothesized that since breast cancer is a heterogeneous disease, gene expression should be different in High Risk breast tumors that may benefit from adjuvant chemotherapy vs. those that are Low Risk and would likely not see any benefit from adjuvant chemotherapy.
Tumor specimens from 78 untreated breast cancer patients with known outcomes were analyzed. The entire human genome was interrogated to determine which genes were most predictive of recurrence at five years, since most recurrences occur within the first five years. As a result, the 70 most significant genes predictive of recurrence risk were identified. With this unbiased gene selection method, the tumor biology told them which genes were most predictive.
MAMMAPRINT INTERROGATES ALL 7 GENOMIC PATHWAYS OF THE METASTATIC CASCADE
The pathogenesis of metastatic disease has 7 critical steps. The first step is growth and proliferation of the primary tumor, which is followed by development of blood vessels into the primary tumor (angiogenesis). The third step, local invasion, introduces the cancer cells into the vascular system. The cancer cells then insert themselves into the vascular compartment through the fourth step, intravasation. After continued survival in circulation (fifth step), the cells must exit out of the circulatory system called extravasation, completing the sixth step. The seventh and final step is where they must adapt to the micro-environment starting the cycle again.
The 70 genes that compose the MammaPrint index are clearly identified within the various steps of the metastatic process.
NO INTERMEDIATES – TRUE BINARY RESULTS
MammaPrint provides a numerical index with a range of -1 to +1, that is overlayed with a binary Low Risk / High Risk clinical classification system. The clinical classification threshold was set by the determination of the largest population of Low Risk patients that can safely withhold chemotherapy.
Low Risk Results
~10% chance (95% CI 4-15) of cancer recurrence within 10 years without any additional adjuvant treatment, either hormonal therapy or chemotherapy
High Risk Results2
~29% chance (95% CI 22-35) of cancer recurrence within 10 years without any additional adjuvant treatment, either hormonal therapy or chemotherapy
VALIDATION
MAMMAPRINT HAS EXTENSIVE CLINICAL VALIDATION
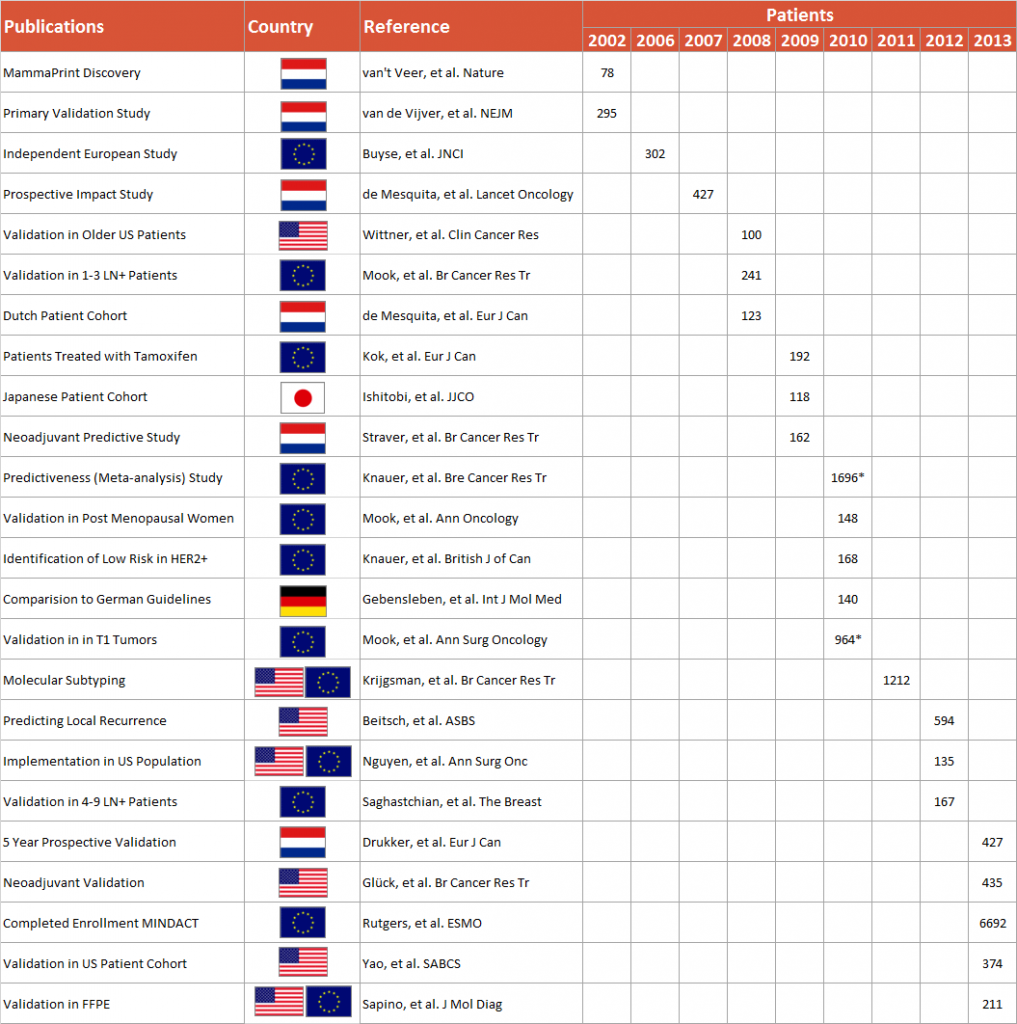
MammaPrint has been independently validated in studies on over 12,000 breast cancer patients, with results published in hundreds of leading peer reviewed medical and scientific journals internationally.
The first validation for MammaPrint, published in the New England Journal of Medicine, was undertaken in a series of 295 consecutive women with breast cancer. The profile was a statistically independent predictor and added to the power of standard clinico-pathologic parameters; HR = 4.6 (95% CI 2.3 – 9.2).
Confidence in the binary results was proven by the independent validation performed by the TRANSBIG Consortium which conducted a study of 302 adjuvantly untreated patients with at least ten years of follow-up.2 Analysis of this data set in line with FDA standards, (conducted in a study by Delahaye et al.) showed that the proportion of patients who remained free from distant metastases at 10 years was 90% in the Low Risk group and 71% in the High Risk group. MammaPrint was found to provide prognostic information beyond what could be determined from patient age, tumor grade, tumor size, and ER status in a population of lymph node-negative patients, none of whom received any adjuvant chemotherapy.
Additional studies have been published proving MammaPrint’s additive prognostic ability in:
– All age groups
– Small primary tumors
– Up to 3 Positive Lymph Nodes (LN+)
– ER +/-
– HER2 +/-
– Ethnic groups
CLINICAL UTILITY
ADJUVANT CHEMOTHERAPY PREDICTIVE DATA7
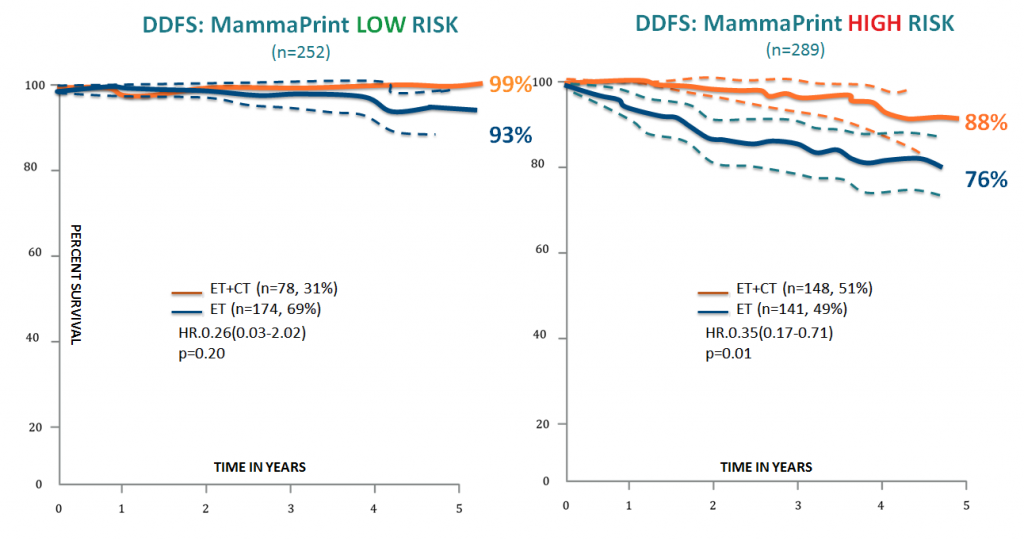
A study published in 2010 by Knauer et al., demonstrated the predictive value of MammaPrint for adjuvant chemotherapy in early stage breast cancer. Patients allocated to High Risk by MammaPrint resulted in a statistical separation in distant disease free survival (DDFS) with a statistically significant p value (p=0.01). In the High Risk group, the endocrine only treated arm had a 76% DDFS, and the endocrine + chemotherapy arm had a 88% DDFS. This resulted in an overall 12% absolute benefit, and a 50% relative benefit overall for this group. In the MammaPrint Low Risk group, no statistically significant benefit for the chemotherapy + endocrine therapy arm vs endocrine therapy only arm was achieved.
Results:
Low Risk = ~10% chance of recurrence = no statistical benefit from chemotherapy
High Risk = ~29% chance of recurrence = statistical benefit from chemotherapy
RASTER STUDY (PROSPECTIVE ANALYSIS)
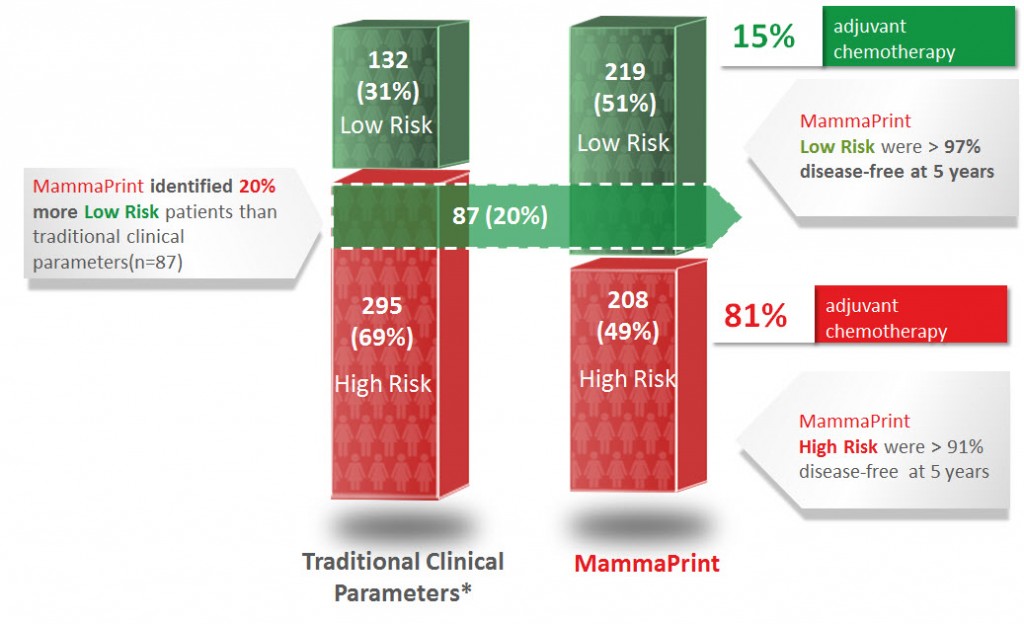
Published in January of 2013, the RASTER study (microarRAy-prognoSTics-in-breast-cancER) proved the ability of MammaPrint to accurately stratify breast cancer patients as either Low Risk or High Risk better than the available traditional clinical parameters. RASTER is the first and only 5 year prospective outcome-based data for any prognostic breast cancer risk of recurrence assay that has been published. The study consisted of 427 patients, that were enrolled at 16 centers, performed in the community setting.
Results:
MammaPrint results were incorporated into the decision making process for patients and physicians with regards to the use of adjuvant chemotherapy
97% of the MammaPrint identified Low Risk patient group, which primarily chose to forego chemotherapy, were disease free after 5 years
MammaPrint identified 20% more Low Risk patients as compared to traditional clinical parameters, and despite this increased allocation in patients, the Low Risk group still had an excellent 97% DRFI
Low Risk MammaPrint result is shown to correlate with excellent outcome
High Risk MammaPrint result is shown to correlate with poorer outcome and higher response to adjuvant chemotherapy
TRANSBIG STUDY
Published in 2006 by Buyse et al., the independent study conducted on behalf of the TRANSBIG consortium which consisted of 302 patients, found that MammaPrint can stratify patients into a binary risk classification of either Low Risk or High Risk, with a statistically significant difference in the probability of metastasis free survival at 10 years.
Results:
Low Risk patients have a ∼10% (95%, CI 4-15) chance of cancer recurrence within 10 years without any adjuvant treatment (either hormonal therapy or chemotherapy)
High Risk patients have a ∼29% (95%, CI 22-35) chance of recurrence within 10 years without any adjuvant treatment (either hormonal therapy or chemotherapy)
MAMMAPRINT IS THE ONLY BREAST CANCER ASSAY BACKED BY PEER-REVIEWED, PROSPECTIVE OUTCOME DATA1
In the first ever prospective clinical study for a breast cancer recurrence assay, RASTER (MicroarRAy PrognoSTics in Breast CancER) confirmed the utility of the MammaPrint 70-gene signature to identify those breast cancer patients that may safely forgo chemotherapy. As compared to standard clincopathological classification, MammaPrint re-stratified 20% of Clinical High Risk patients to Low risk. 97% of this Low Risk patient group which primarily chose to forgo chemotherapy, were disease free at 5 years.